Vaccination for Human Papillomavirus (HPV) (Indicator 22)
Updated
The indicator describes the following: Vaccination coverage for human papilloma virus (HPV) vaccine.
A vaccine against human papillomavirus (HPV) is included in the Norwegian Childhood Immunisation Programme and is given at 12 years (7th grade). The vaccine has been offered to girls since 2009 and to boys since 2018. In addition there was a limited catch-up programme from 1 November 2016 to 30 June 2019 for young women born in 1991 or later. In this programme, women who had never been offered or had previously declined the offer in the Childhood Immunisation Programme were offered the vaccine for free. The Norwegian Immunisation Registry (SYSVAK) provides statistics of the vaccination coverage for HPV vaccination.
Results
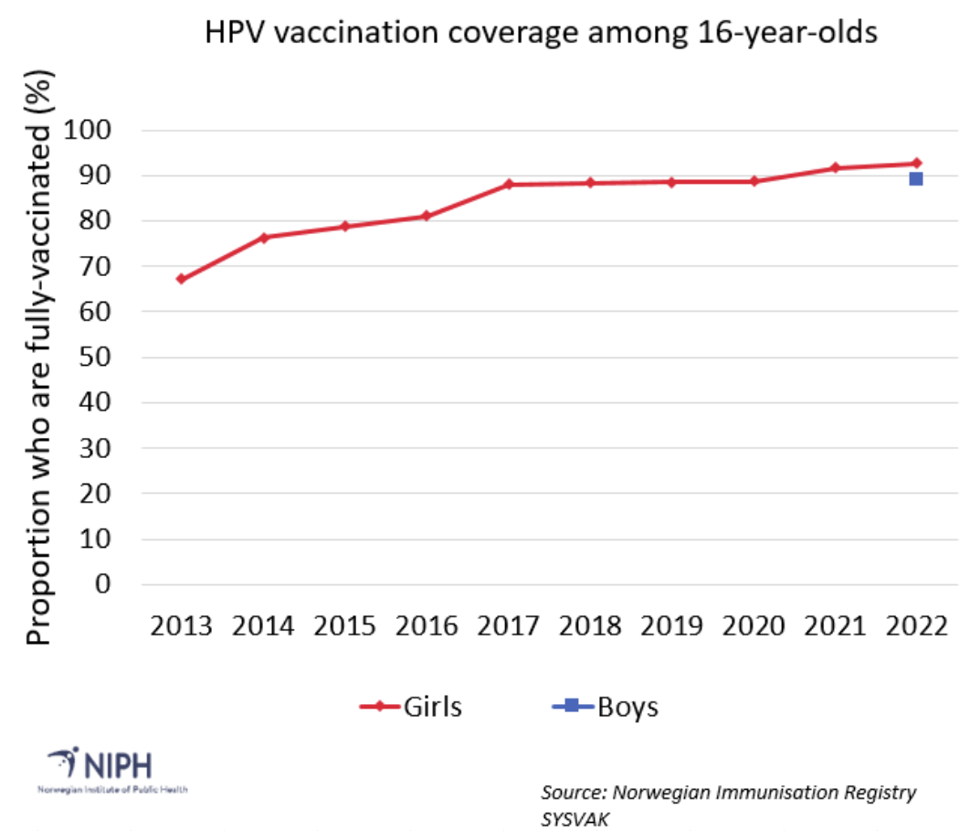
The official vaccination coverage for the HPV vaccine is given for 16-year-olds. The vaccination coverage among 16-year-old girls who are fully vaccinated increased from 67 per cent to 93 per cent between 2013 and 2022 (see figure 1). Among 16-year-old boys in the first cohort that was offered HPV vaccine through the childhood immunisation programme (boys born in 2006) 89 per cent are fully vaccinated. The proportions of vaccinated boys and girls are monitored continuously and for the 2010 cohort, 93 per cent of girls and 91 per cent of boys have received at least one dose of HPV vaccine (as of August 2023). 59 per cent of young women born between 1991 and 1996 have received at least one dose of HPV vaccine during the time-limited catch-up programme.
|
Table accompanying Figure 1. Vaccination coverage for fully-vaccinated 16-year-olds, as a percentage |
||||||||||
|
|
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
|
Girls 16 years |
67 |
76 |
79 |
81 |
88 |
88 |
89 |
89 |
92 |
93 |
|
Boys 16 years |
- |
- |
- |
- |
- |
- |
- |
- |
- |
89 |
Data source: Norwegian Immunisation Registry (SYSVAK)
The data source for this indicator is the Norwegian Immunisation Registry (SYSVAK). A description follows below.
Description
The Norwegian Immunisation Registry (SYSVAK) maintains an overview of the individual's vaccination status and vaccination coverage in Norway.
The Norwegian Childhood Immunisation Programme includes a range of different vaccines recommended by the health authorities for children and adolescents.
The Norwegian Institute of Public Health is responsible for procuring the vaccines for the Norwegian Childhood Immunisation Programme and distributing them to the municipalities. Vaccination takes place at well-baby clinics and through the school health service and is free of charge. All administered vaccines are registered at individual level in SYSVAK.
The Norwegian Childhood Immunisation Programme publishes an annual report that includes vaccination coverage, reported cases of diseases for which vaccination is provided through the immunisation programme, and the type of vaccines that have been used.
Effect measure
- Vaccination coverage for girls and boys aged 16 years who are fully vaccinated with the HPV vaccine, as a percentage.
The figures apply to those who are fully vaccinated, i.e. they have received the recommended number of doses with the recommended intervals between doses. In the Childhood Immunisation Programme, the HPV vaccine was administered in a three-dose schedule from autumn 2009 until autumn 2017. Since autumn 2017, the vaccine has been administered in a two-dose schedule.
The HPV vaccine is offered at 12 years (7th grade). The official vaccination coverage is calculated for 16-year-olds.
National adaptation to global indicator definition
WHO’s definition of the indicator
Indicator 22. Availability, as appropriate, if cost-effective and affordable, of vaccines against human papillomavirus, according to national programmes and policies.
National adaptation
The WHO indicator applies to vaccine availability. The Norwegian indicator includes vaccination coverage.